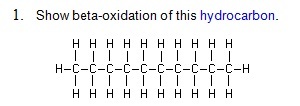Introduction
Bacteria vary in their nutritional requirements. Some cause food spoilage, others metabolize toxic substances such as heavy metals, sulfur, petroleum, PCBs and mercury. The use of bacteria to eliminate pollutants is called bioremediation. Unlike some forms of environmental clean-up where dangerous substances are removed from one place only to be dumped in another, bacterial clean-up eliminates the toxic substance, and often returns a harmless or useful substance to the environment.
An investigation of hydrocarbon degrading bacteria will be investigated. A hydrocarbon is a compound that contains only carbon and hydrogen atoms. The carbon chains can be straight, branched, or rings. Several naturally occurring bacteria in the genus Pseudomonas are able to degrade oil for their carbon and energy requirements. In the presence of air, they remove two carbons at a time, in a process called beta-oxidation, from a large petroleum molecule. These bacteria degrade the oil too slowly but can be speed up by bioaugmentation, the introduction of nitrogen and phosphorous plant fertilizers to increase the number of oil-degrading bacteria.
In this exercise, hydrocarbon-degrading bacteria in various samples are enriched using the sheen screen method developed by Ed Brown in Valdez , Alaska
OBJECTIVES
1. To be able to understand bioremediation and the principles behind.
2. To demonstrate enrichment of oil degrading bacteria
METHODOLOGY
A. Oil Spill
1. Prepare 2 jars for the set-up with label A and B. To each jar, add approximately 5.0 mL distilled water and 4-5 drops of oil. Record carefully the general appearance, color, and texture of the oil.
2. Add 0.5 g of soil to Jar A and nothing to Jar B.
3. Screw the lid on both jar and invert several time to mix
4. Incubate the jars with the lids loosened a half-turn at 30°C
5. Invert the jars once or twice daily to increase the dissolved oxygen content in the water and to mix the microbes with the oil.
6. Observe jars every 24 hr for 3-4 days. Make careful observations of the general appearance, color, texture, and turbidity of the water.
B. Bioaugmentation
1. Prepare 2 jars for the set-up with label A and B. To each jar, add approximately 5.0 mL distilled water and 15-20 drops of oil. Record carefully the general appearance, color, and texture of the oil.
2. Add a pinch of fertilizer over the entire oil layer in each jar.
3. Add 0.5 g of soil to A and nothing to B.
4. Screw the lid on both jar and invert several time to mix
5. Incubate the jars with the lids loosened a half-turn at 30°C
6. Invert the jars once or twice daily to increase the dissolved oxygen content in the water and to mix the microbes with the oil.
7. Observe jars every 24 hr for 3-4 days. Make careful observations of the general appearance, color, texture, and turbidity of the water.
Suggested Activities
1. Use sand and gravel in a Petri plate or shallow pan instead of a liquid culture to simulate an oil spill. Test the rates of degradation with (a) no inoculum; (b) a soil inoculum; (c) soil and fertilizer.
2. Change the hydrocarbon to compare rates of degradation.
3. The jars labeled B are enrichments for hydrocarbon-degrading bacteria. Use samples from these jars as inocula for the experiments on Bioremediation of Gasoline and Bioremediation of Oils.
RESULTS AND DISCUSSION
Guide Questions
1. How can you tell whether the oil was degraded?
- What is the limiting factors in Part A?
- How could the oil-degrading bacteria you isolated be used to clean up an oil spill?
RESULTS:
1. Show beta-oxidation of this hydrocarbon.
2. Here are the formulas of two detergents that have been manufactured: Which of these bacteria would readily degrade? Why?